- Phone: +91 9022447976 | +91 9106194096
- 24x7 Hours Working Hospital
- info@urbanhealhospital.com | drkartik@urbanhealhospital.com

IVF means in vitro fertilization. In vitro means in the laboratory ( outside the body) and fertilization refers to conception (joining of a woman’s egg and a man’s sperm in a laboratory dish) . This technology enhances the likelihood of conception for couples facing infertility issues. IVF may be considered when other treatment methods have been unsuccessful, and in certain cases, such as bilateral fallopian tubal disease, it may be the most viable option for achieving success. Beginning the IVF process can be an exciting venture for couples.
When is IVF treatment recommended?
Here are some reasons why IVF treatment may be recommended:
What screening procedures are conducted prior to initiating the IVF treatment?
Ovarian Stimulation:
Normally, your ovaries produce one egg per month during a regular cycle. An IVF cycle uses medicine to stimulate follicle growth, which is the process by which the eggs grow, in an effort to produce more eggs. Injections are typically used to administer this medicine. You will need to come to the clinic for ultrasound scans in order to track the development of follicles during ovarian stimulation. Stimulation is finished when your doctor certifies that the follicles have grown to their ideal size.
Follicular Puncture (Oocyte Pickup): To encourage egg maturation, a trigger shot is given once the follicles have grown to their ideal size. Human chorionic gonadotrophin (hCG) injections are typically used as the trigger shot. The goal is to retrieve the eggs 34–36 hours after the trigger shot. The trigger shot must be administered at the precise time allotted because it will determine the date and time of the oocyte (egg) retrieval. This method is for day cases. Using state-of-the-art ultrasound technology, the doctor will do a transvaginal oocyte (egg) collection under local anesthesia, or in certain situations, general anesthesia.
Oocyte Fertilization and Embryo Culture: The semen sample is taken and ready for use on the day of the oocyte retrieval process. In a procedure known as insemination, our embryologists will combine the eggs and sperm in culture media dishes so that natural fertilization can occur.
Approximately 17 hours after the insemination (IVF), the oocytes are placed in our specialist incubators to be evaluated for fertilization. The “RI-Witness” method is employed at our Clinic to ensure accurate sperm and egg identification. The “Xiltrix system” keeps an eye on every incubator in the lab to make sure the embryos are kept in a secure environment.
Embryo Transfer: Every day, the embryos’ progress is assessed. When to transfer the embryo(s) to get the best possibility of a successful pregnancy is a decision made by the doctor and the embryologist. The ideal day for embryo transfer is determined by taking into account the following factors:
With the use of a soft, flexible catheter, the embryo(s) is/are transplanted into the uterus under ultrasound guidance. It is a straightforward, painless operation that doesn’t require any anesthetic or medication. It isn’t suggested to lie in bed after the embryo transfer. After the embryo transfer process, you can contact our team of professionals if you have any questions or concerns.
 Controlled Ovarian Hyperstimulation (COH) involves inducing the development of more ovarian follicles than usual in a woman’s menstrual cycle, achieved with fertility medications. As multiple follicles develop, the number of viable eggs also increases, thereby enhancing the likelihood of conception.
Controlled Ovarian Hyperstimulation (COH) involves inducing the development of more ovarian follicles than usual in a woman’s menstrual cycle, achieved with fertility medications. As multiple follicles develop, the number of viable eggs also increases, thereby enhancing the likelihood of conception.
What is the process of COH?
The main goal of COH is to optimize and improve ovarian function. Prescribed fertility medications regulate endocrine functions, allowing doctors to anticipate hormonal fluctuations accurately, which would be difficult otherwise. This oversight aids in assessing egg quality, enabling doctors to recommend the most appropriate artificial insemination technique, such as IUI or IVF, to the patient.
Does COH improve the likelihood of conception?
COH is seen as an additional support for individuals undergoing IUI, IVF, and similar methods to conceive. Research indicates that when COH precedes an IUI procedure, there is an enhanced chance of conception.
Despite the decreased chances of pregnancy as women age, COH combined with IUI or IVF has resulted in successful pregnancies for women of advanced maternal age.
 When is ICSI suggested?
When is ICSI suggested?
ICSI is advised under the following circumstances:
What is the procedure for performing ICSI?
The process of ICSI follows similar steps to IVF:
What is the success rate of an ICSI procedure?
ICSI is utilized to address severe male infertility by facilitating the fertilization of the sperm and egg. It enhances the likelihood of achieving a successful pregnancy for couples affected by male factor infertility or previous IVF fertilization failures. ICSI represents a significant advancement in assisted reproductive technology, providing men with severe male factor infertility the chance to conceive biological children.
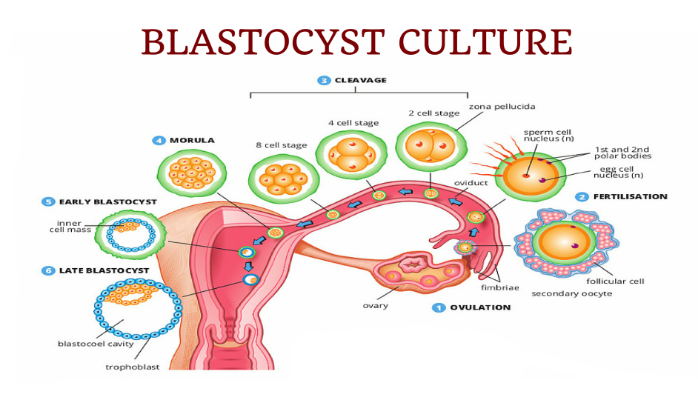 After fertilization, the embryo which develops from the fertilized egg goes through various stages of development. A normal pattern of embryo development is outlined below:
After fertilization, the embryo which develops from the fertilized egg goes through various stages of development. A normal pattern of embryo development is outlined below:
Blastocyst quality is determined by evaluating the external appearance of the blastocyst. As previously described the blastocyst has 2 distinct cell layers. The outer rim of cells referred to as the trophectoderm which develops into the placenta and the inner cell mass which is made up of stem cells which ultimately develop into the baby. Grading of the blastocyst is an imperfect science. Blastocysts that have been assigned a high grade/ top quality do not necessarily result in pregnancy. However, the best embryos will survive to the blastocyst stage and by virtue of the fact that they survived to day 5, day 6 indicate that they have the best implantation potential.
Blastocyst Transfer
The last step involves transferring the blastocyst to the uterus using a soft, flexible catheter guided by ultrasound. After insertion, the catheter is carefully removed, and the embryologist confirms that the blastocyst has been successfully transferred. Resting in bed after the procedure is not recommended.
 Intrauterine Insemination (IUI) is a form of artificial insemination used to address infertility concerns.
Intrauterine Insemination (IUI) is a form of artificial insemination used to address infertility concerns.
How is IUI done?
During Intrauterine Insemination (IUI), processed sperm is injected into the woman’s uterus via the cervix during her menstrual cycle. This procedure can occur naturally or be controlled, with ovulation stimulated to produce a few eggs rather than the usual one or two. The process is typically painless.
The timing of IUI is carefully managed, with follicle growth monitored using ultrasound to minimize the risk of multiple pregnancies and determine the optimal time for the procedure. Once follicles reach the appropriate size, human chorionic gonadotropin (hCG) is administered to trigger ovulation. This allows the eggs to mature within the follicles, ready for release approximately 36 hours later. The IUI is scheduled accordingly, typically within 24 to 36 hours after the trigger shot.
When is IUI suggested?
IUI is typically recommended in the following scenarios:
Further IUI treatments are usually not recommended if there is no pregnancy after three insemination attempts.
 An alternate technique for choosing embryos for IVF transfer is preimplantation genetic testing for aneuploidies (PGT-A), formerly known as preimplantation genetic screening (PGS). With IVF, this test finds the preimplantation embryos that have a normal chromosomal complement, or euploidy. PGT-A filters out embryos with an aberrant chromosomal copy number, increasing the success rate of conception.
An alternate technique for choosing embryos for IVF transfer is preimplantation genetic testing for aneuploidies (PGT-A), formerly known as preimplantation genetic screening (PGS). With IVF, this test finds the preimplantation embryos that have a normal chromosomal complement, or euploidy. PGT-A filters out embryos with an aberrant chromosomal copy number, increasing the success rate of conception.
What are the reasons to do PGT-A?
A healthy infant can be born into an embryo that implants and matures to full term if its chromosomal copy number is normal. Under a microscope, it is typically challenging to visually recognize the normal embryo. An embryo’s normal appearance does not guarantee that it is devoid of genetic abnormalities. Therefore, PGT-A aids in the selection of embryos with a normal number of chromosomes.
Who should consider PGT-A?
When and how is PGT-A conducted?
In this process, IVF-produced embryos are tested, and the ones with the appropriate amount of chromosomal material are chosen and transferred.
To what extent is PGT-A safe?
This process is safe. Even after the embryo biopsy, the process should be managed carefully by a skilled embryologist so that the embryo develops normally.
Does PGT-A evaluate all genetic diseases?
Only numerical chromosomal number variations and other genetic material imbalances, such as deletions (where a chromosome is missing) and duplications (where a chromosome is duplicated), can be evaluated by PGT-A testing.
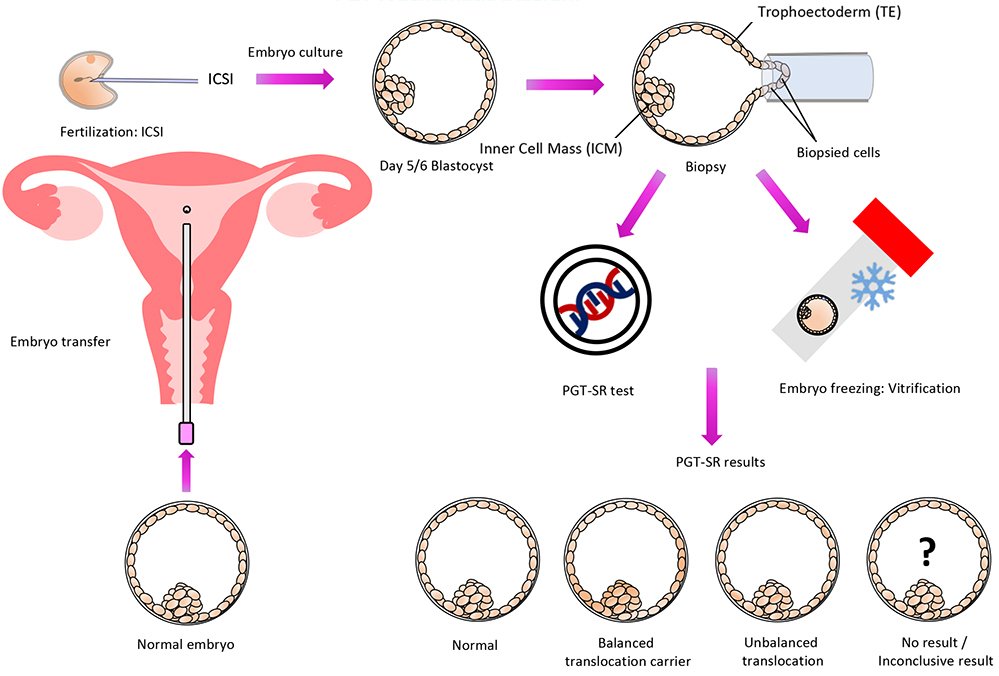 With the use of a genetic test called PGT-SR, chromosomal structural rearrangements that are inherited can be found in embryos before they are transferred, increasing the likelihood of a healthy pregnancy.
With the use of a genetic test called PGT-SR, chromosomal structural rearrangements that are inherited can be found in embryos before they are transferred, increasing the likelihood of a healthy pregnancy.
Since affected spouses are typically healthy and are unaware of the chromosomal structural rearrangement they have, this testing is frequently thought of following many miscarriages or the birth of an offspring with chromosomal abnormalities. They simply have a higher chance of producing embryos with the wrong number or shape of chromosomes because of their disease.
For whom is PGT-SR appropriate?
People who have been identified by specialized genetic testing such as karyotyping as having a chromosomal rearrangement (e.g., inversion, reciprocal translocation, Robertsonian translocation, etc.) may benefit from the PGT-SR test. By transferring embryos with an accurate chromosomal content, it increases the likelihood of a successful pregnancy and enables the detection of inaccurate chromosomal copy numbers (i.e., gains or losses of chromosomes or sections of chromosomes).
How is PGT-SR done?
In this process, IVF-produced embryos are tested, and the ones with the appropriate amount of chromosomal material are chosen and transferred.
What are the advantages of PGT-SR?
 Preimplantation genetic diagnosis (PGD) was the previous term for preimplantation genetic testing (PGT-M) for monogenic or single gene disorders. A diagnostic technique called PGT-M is used to identify single-gene illnesses in individuals who have a high chance of passing on genetic abnormalities to their kids. This test helps to reduce the risk of having genetic diseases in their kids.
Preimplantation genetic diagnosis (PGD) was the previous term for preimplantation genetic testing (PGT-M) for monogenic or single gene disorders. A diagnostic technique called PGT-M is used to identify single-gene illnesses in individuals who have a high chance of passing on genetic abnormalities to their kids. This test helps to reduce the risk of having genetic diseases in their kids.
Why should one perform PGT-M?
A healthy pregnancy can be conceived by couples with the use of this test. The entire procedure is performed before a female conceives. PGT- M:
For whom the PGT-M appropriate?
What are the requirements to complete PGT-M?
A genetic condition is caused by a mutation that modifies the DNA sequence, which changes how a gene functions.
When and how is PGT-M conducted?
Testing IVF-produced embryos and choosing and transferring those with the appropriate quantity of chromosomal material are all part of this process.
Is PGT-M safe to perform?
PGT-M can be performed safely. An experienced embryologist must do the process. After the embryo biopsy, normal development of the embryo is shown.
 Did you know?
Did you know?
The likelihood of a successful pregnancy increases significantly when the precise cause of a man’s infertility is identified. Treatments for infertility can involve anything from medication to surgery to fix the issue.
Semen Analysis
The microscopic inspection of sperm shape, quantity, and motility is known as semen analysis. In a lab, it is performed on recently ejaculated semen. This analysis can be used to determine male infertility.
It is done at least twice to examine the semen. This is done to properly check for fertility. The quality of the male semen varies throughout samples. Even when the male is fertile, illnesses, fevers, and infections can occasionally cause the male’s sperm quality to decline for a few months.
When is a semen analysis done?
An analysis of the male partner’s semen is part of the testing that takes place when a couple is evaluated medically for infertility. This analysis can also be used to verify that the vasectomy was successful.
In this analysis, which parameters are checked?
How is the semen collected for testing done?
How do you evaluate the sperm?
To get the most accurate results, the semen is stored at room temperature. Samples collected from patients who are agitated or ill may have a negative impact on the results of the test. Following the collection of semen, the sperm is analyzed, and skilled professionals perform a number of tests to get results.
WHO Guidelines for Semen Analysis (2021):
What conclusions can be drawn from a semen analysis?
Semen analysis cannot guarantee fertility because infertility can have other causes. Even with an extremely low sperm concentration, the couple may still be able to conceive naturally. The likelihood of conception can be affected by a number of factors, including the frequency and timing of the couple’s intercourse, the number of times they have attempted to conceive, and the age and fertility of the female partner.
Microscopic testicular sperm extraction (Micro-TESE) is a procedure that involves extracting sperm directly from the testicular tissue of a man, aiming to obtain high-quality sperm, sufficient for fertilization, while minimizing damage to the reproductive organs.
Who needs micro-TESE?
Micro-TESE is recommended for individuals experiencing abnormal sperm production as a result of factors such as prior testicular surgery, medical interventions, genetic conditions, or other male infertility issues.
How is micro-TESE performed?
Micro-TESE is performed on male patients under general anesthesia. A surgical incision is made in the testis to locate and examine the seminiferous tubules, particularly focusing on swollen tubules containing sperm, using a high-power operating microscope. Once the procedure is completed, the testis is closed with fine dissolvable sutures. Patients typically recover within 1 to 2 weeks and can resume normal activities. If sperm are found during the procedure, they can be immediately extracted for fertilization or preserved for future use in reproductive treatments such as ICSI.
What is the process of COH?
The main goal of COH is to optimize and improve ovarian function. Prescribed fertility medications regulate endocrine functions, allowing doctors to anticipate hormonal fluctuations accurately, which would be difficult otherwise. This oversight aids in assessing egg quality, enabling doctors to recommend the most appropriate artificial insemination technique, such as IUI or IVF, to the patient.
Does COH improve the likelihood of conception?
COH is seen as an additional support for individuals undergoing IUI, IVF, and similar methods to conceive. Research indicates that when COH precedes an IUI procedure, there is an enhanced chance of conception.
Despite the decreased chances of pregnancy as women age, COH combined with IUI or IVF has resulted in successful pregnancies for women of advanced maternal age.
The procedure known as percutaneous epididymal sperm aspiration, or PESA, is used to extract sperm from the epididymis, which is the tube that joins a testicle to the vas deferens in the male reproductive system.
What are the benefits of PESA?
PESA does not require making a surgical incision in the scrotum. This approach is easy to reproduce and is quick, easy to use, and economical.
WhatsApp us